1987
TAUNS Laboratories, Inc. was founded in Numazu (Shizuoka) to conduct research and development of in vitro diagnostics, research reagents, various analytical reagents and related systems.
Tokyo office was established and began operations.
1994
A new head office building was constructed in Kozuwa (Numazu, Shizuoka).
2001
A new diagnostic manufacturing factory (Numazu Factory) was constructed in Kozuwa (Numazu, Shizuoka).
2002
The R&D department was spun off to form BL Co., Ltd.
2007
Our platinum-gold colloid manufacturing process was patented.
A new head office building was constructed in Futaba-cho (Numazu, Shizuoka). 
2008
TAUNS started production and sales of Capilia O157, a diagnostic test kit for E. coli O157 infection, and Capilia VT, a diagnostic test kit for enterohemorrhagic E. coli infection.
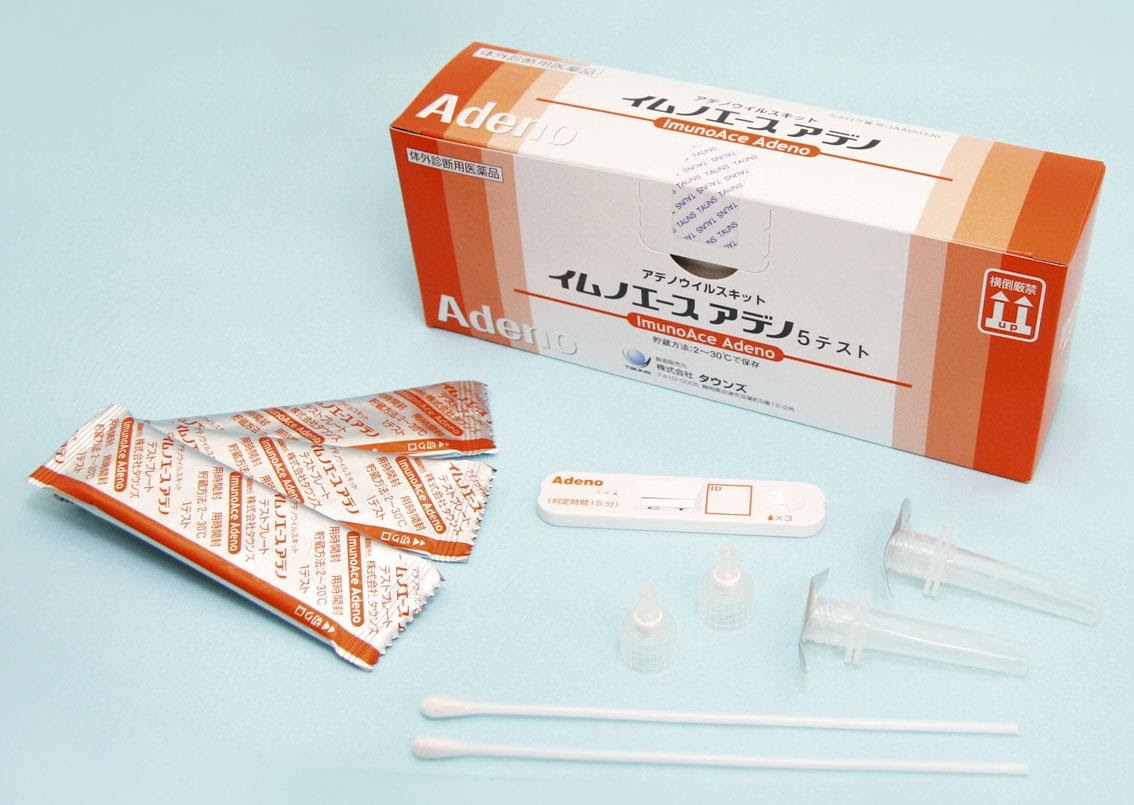
ImunoAce Adeno, a rapid diagnostic test kit for adenovirus infection, was launched in Japan. It was the world’s first rapid diagnostic test kit adopting the latest platinum-gold colloid nanotechnology.
ImunoAce Flu, a rapid diagnostic test kit for influenza virus infection, was launched in Japan.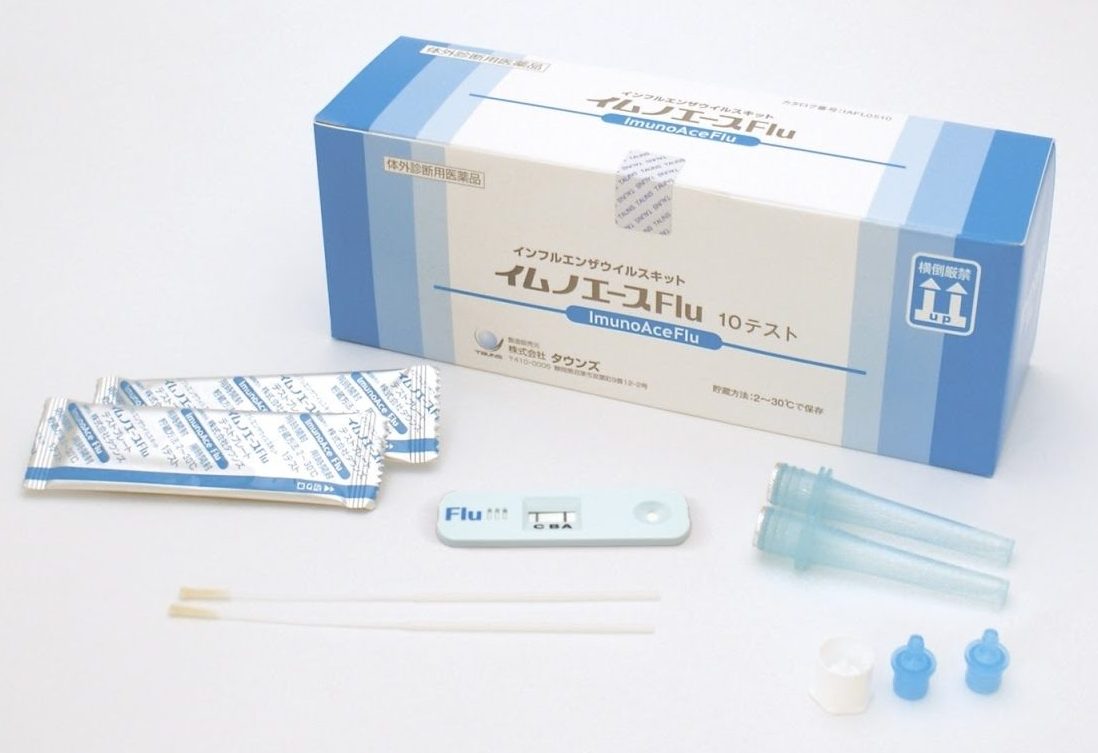
2010
Line Judge FluA/pdm, a research reagent, was launched in Japan.
2011
Capilia MAC Ab ELISA, a diagnostic test kit for MAC pulmonary disease, was launched in Japan.
2012
Kamishima Factory was established in Kamishima (Izunokuni, Shizuoka).
Capilia TB-Neo, a diagnostic test kit for tuberculosis, was launched in Japan.
Head office was relocated to Kamishima (Izunokuni, Shizuoka).
ISO 13485 certification was acquired.
ImunoAce RSV Neo, a rapid diagnostic test kit for RS virus infection, was launched in Japan.
2013
ImunoAce Strep A, a rapid diagnostic test kit for GABHS infection, was launched in Japan.
2015
ImunoAce Mycoplasma, a rapid diagnostic test kit for mycoplasma infection, was launched in Japan.
2016
ImunoAce hMPV, a rapid diagnostic test kit for human metapneumovirus infection, was launched in Japan.
TAUNS merged with BL Co., Ltd. and integrated its R&D department.
2020
ImunoAce Flu/RSV, a rapid diagnostic test kit for influenza virus and/or RS virus infection, was launched in Japan.
Shimizu-cho Office/R&D Center was established in Shimizu-cho (Sunto-gun, Shizuoka).
ImunoAce Strep A Neo, a rapid diagnostic test kit for GABHS infection, was launched in Japan.
ImunoAce SARS-CoV-2, a rapid diagnostic test kit for SARS-CoV-2 infection, was launched in Japan.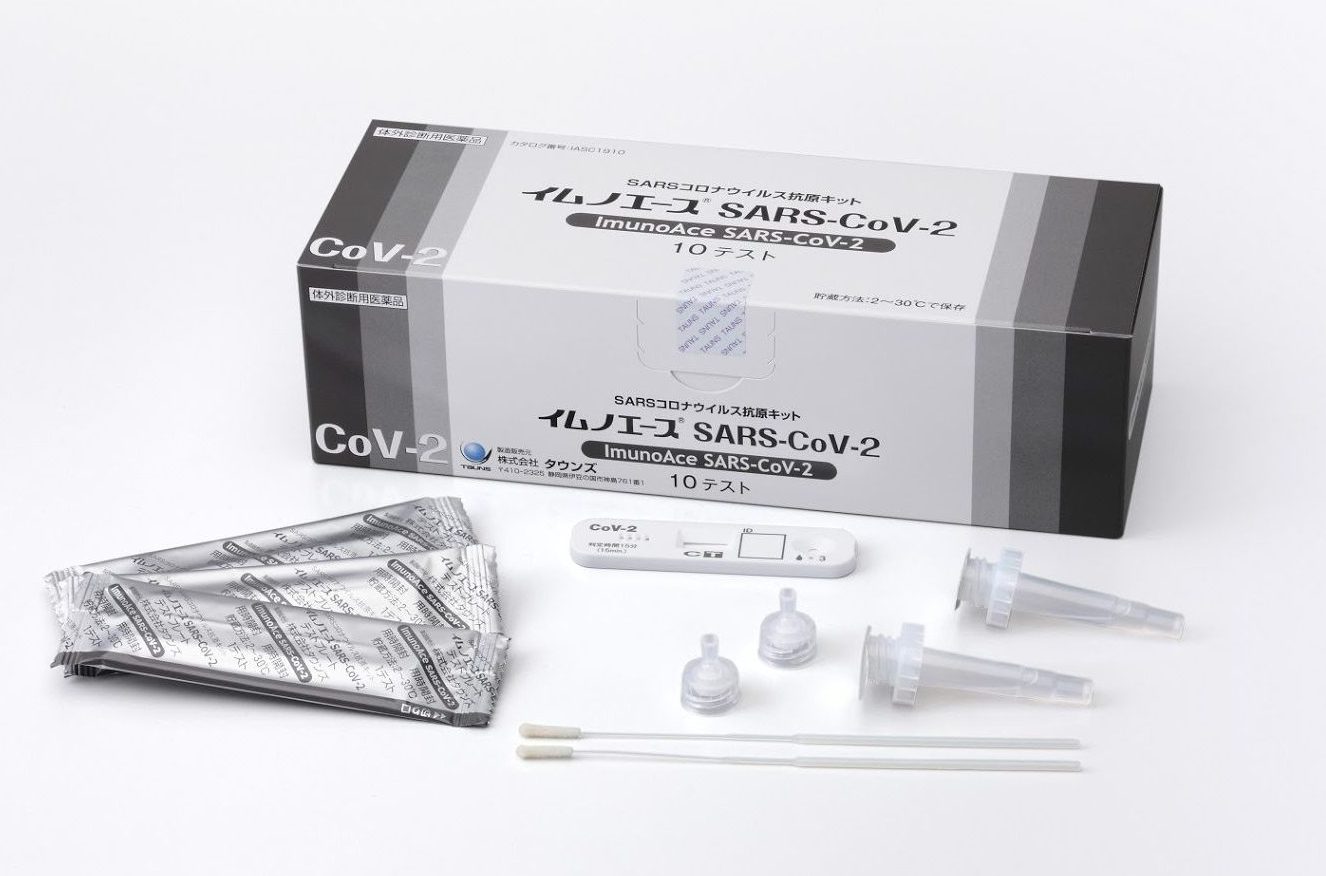
ImunoAce Noro, a rapid diagnostic test kit for norovirus infection, was launched in Japan.
2021
ImunoAce SARS-CoV-2 Ⅱ, a rapid diagnostic test kit for SARS-CoV-2 infection, was launched in Japan.
2022
ImunoAce SARS-CoV-2 Saliva, a rapid diagnostic test kit for SARS-CoV-2 infection, was launched in Japan.
ImunoAce SARS-CoV-2/Flu, a rapid diagnostic test kit for SARS-CoV-2 and influenza virus infection, was launched in Japan.
2023
TAUNS developed a zinc quantification kit “ACCUREAD Zn” and
a spectrophotometer “ACCUREAD” (distributed by Nobelpharma Co., Ltd.).
2024
ImunoAce SARS-CoV-2 Ⅲ, a rapid diagnostic test kit for SARS-CoV-2 infection, was launched in Japan.
TAUNS listed its shares on the Standard Market of the Tokyo Stock
Exchange.